Science: Palladium-catalyzed selective synthesis of BCB!
2024-08-02
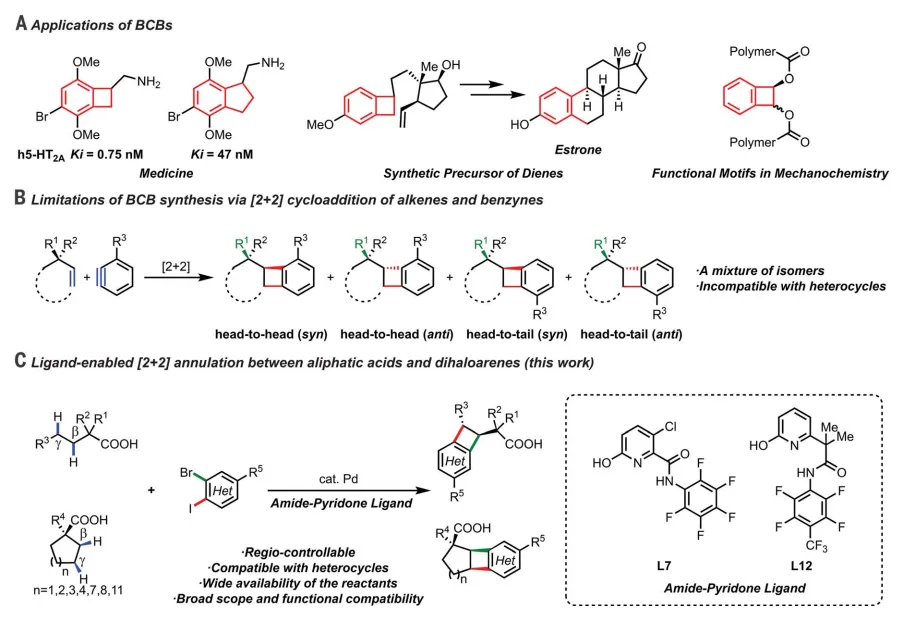
Research background B- enzocyclobutenes(BCBs) represent an important class of rigid four-membered carbon rings that exist in natural products and show great potential as therapeutic molecular scaffolds, multifunctional syntheses and functional motifs in material science and mechanization. The BCB-based rigid and three-dimensional pharmacophore led to the discovery of ivabradine, an FDA-approved drug for the treatment of heart failure and heart-related chest pain. In addition, BCB analogs of psychoactive 2C-B have better affinity for the receptor than the conformationally flexible parent compound and benzocyclopentane analogs.

research background
B- enzocyclobutenes(BCBs) represent an important classrigid quaternary carbon ring, present in natural products, show great potential as therapeutic molecular scaffolds, multifunctional syntheses and functional motifs in material science and mechanization. Based onThe rigidity and three-dimensional pharmacophore of BCB led to the discovery of ivabradine, an FDA-approved drug for the treatment of heart failure and heart-related chest pain. In addition, BCB analogs of psychoactive 2C-B have better affinity for the receptor than the conformationally flexible parent compound and benzocyclopentane analogs.
Key issues
however,The synthesis of BCBs remains problematic:
1. Regioselectivity of the cycloaddition reaction to control the synthesis of BCBs has not been achievedAt present, olefins and benzene alkynes.[2 2] The cycloaddition reaction is one of the most common synthetic routes for BCBs. However, controlling the regioselectivity of the cycloaddition reaction is an unsolved problem.
2. It is urgent to develop selective ways to prepare various rigid carbon rings.ExistingBCB synthesis methods have limitations in scope and efficiency. Using these reactions to synthesize heterocyclic BCB with medicinal value is challenging. It is necessary to find alternative and more selective ways to prepare rich C(sp3) characteristics of various rigid carbon rings.
new ideas
in view of this,Scripps Institute Yu JinquanOthersA palladium-catalyzed double of two adjacent methylene units in a carboxylic acid is reportedC- H activation, under the action of bidentate amide-pyridone ligands,By involving only(Two C- H bonds and two aryl halide bonds) in the form of s bond [2 2] cycloaddition reaction, realizing the regio-controllable synthesis of BCBs. A wide range of cyclic and acyclic fatty acids as well as dihalo-heterocyclic aromatic hydrocarbons are compatible, resulting in a variety of functionalizedBCBs and hetero-BCBs, present in drug molecules and biologically active natural products.

Technical scheme:
1. Clarified the reaction development processsubjectΒ-/γ-Inspired C- H deuteration, the author explored the development of a sequential double C- H coupling process with ortho-dihaloarenes to construct BCB scaffolds. The feasibility of the reaction was confirmed by experiments and the separation efficiency was improved.
2. The cyclic acid substrate range was evaluatedThe authors evaluated[2 2] The substrate range of the cyclization reaction indicates that multiple cyclic fatty acids can provide cis-BCB products as a single product, and larger cyclic fatty acids produce trans-BCB products as the main isomer.
3. Assessed the acyclic acid substrate rangeAuthor discovers six-membered chelating amideThe-pyridone ligand is the most effective ligand for linear substrates, providing a variety of trans-BCB, and also demonstrating the synthetic processing of many benzocyclobutyric acid products.
4. Exploring the range of two halogenated aromatic substratesThe author proved that the substituents at different positions are compatible with the catalytic system, and halogens such as fluorine, chlorine and bromine are compatible and can provide the requiredBCB。
5. Study on the applicability and mechanism of heteroaromatic hydrocarbons and complex moleculesThe authors found that heterocyclic compounds such as pyrrole, thiophene and furan were well tolerated, twoThe sequential activation of the C- H bond and the two aryl halide bonds has an unexpectedly exclusive regioselectivity.
Technical advantages:
1. Pd-catalyzed BCB synthesis was successfully realized, and specific area control was realized.Author by amideThe cyclization reaction of fatty acids with dihaloarenes achieved by-pyridone ligands has successfully achieved Pd-catalyzed BCB synthesis, in which specific domain control is achieved by the difference between aryl iodide and bromine.
2. Development of a simple, efficient and universal synthesis method for BCBsThe direct use of abundant and structurally diverse non-cyclic acids and cyclic acids as substrates without pre-functionalization greatly expands the acquisition of variousPathways to BCB, including heterocyclic BCB scaffolds.
Technical Details
reaction developmentIn the methylene group of the dicarboxylic acidIn the study of the mechanism of C- H lactonization reaction, it was observed that 7-ethoxy-7-oxoheptanoic acidΒ,γ-C-H deuteration. This observation prompted the authors to investigate whether a sequential double C- H coupling process with ortho-dihaloarenes could be developed to construct BCB scaffolds. Use1a(1-propyl -1-cyclopentane carboxylic acid) and2a(dihaloarene coupling1-bromo-4-chloro-2-iodobenzene) and Ag2CO3As a halide scavenger with a series of pyridine-pyridone ligands test the feasibility of this transformation, and the results show that the required3a(BCB) does form as a single isomer. The preliminary results of the pyridine-pyridone ligand show that more effective ligands are needed. The author focuses on the development of alternatives to the pyridine arm of the scaffold. A class of bidentate ligand perfluoroaniline with both pyridone and electron-deficient amide is synthesized. Through extensive structural adjustment, the ligandL7determined as the optimal ligand90% isolated yield provides3a.
Figure[2 2] Bis-methylene C- H bond functionalization cyclization reaction
Cyclic acid substrate rangeThe authors evaluated[2 2] The substrate range of the cyclization reaction. including five-membered rings (1a-1l), six-membered ring (1m-1t), seven-membered ring (1u-1v) and eight-membered rings (1w) is compatible with a variety of cyclic fatty acids, and can provide cis BCB products as a single product regioisomer (3a-3w). The larger cyclic fatty acids produce the trans BCB product as the major isomer. When the yield is relatively low, the remaining unreacted fatty acid reactant is recovered. LigandL7AllowPd loading is reduced from 10 to 5 or 1% while maintaining dihaloaromatics2aand acid[2 2] synthesis of cyclization reaction useful yield.
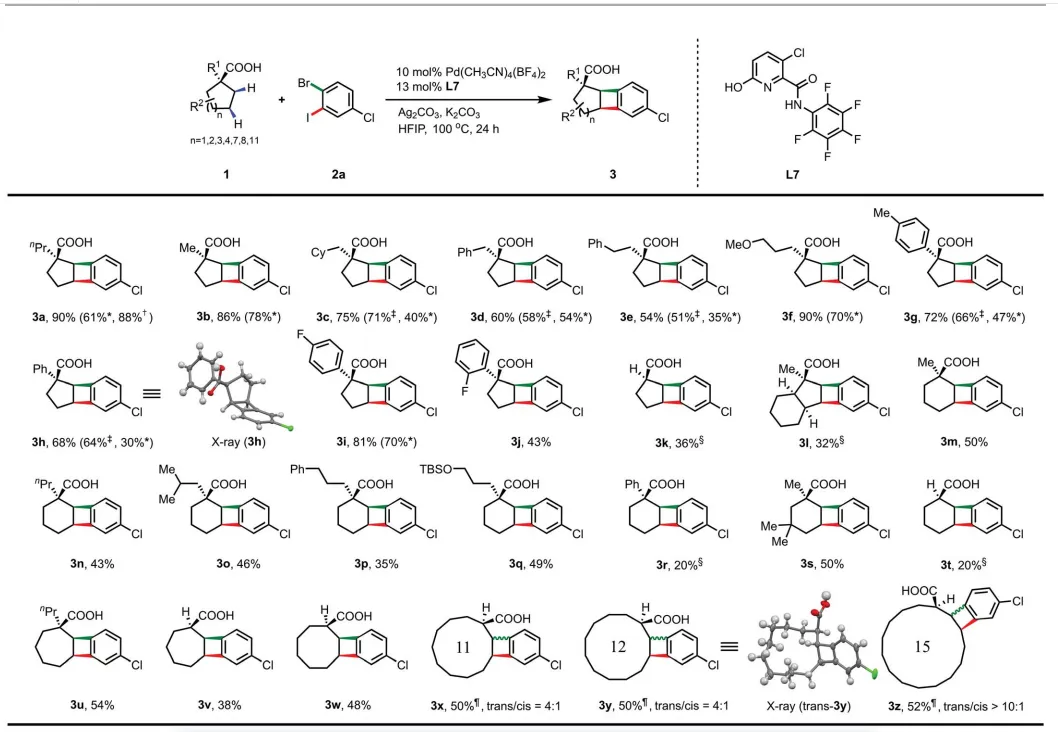
Figure[2 2] Cyclic fatty acid range of cyclization reaction
Acyclic acid substrate range
acyclic fatty acid4aInL7produce only low yields in (2%). six-membered chelating amide-pyridone ligandL12It becomes the most effective ligand for these linear substrates, providing a variety of trans-BCBs. Various functional groups such as methoxy, chloro, phosphonate and substituted aryl groups are compatible. The authors also demonstrate the synthetic processing of a number of benzocyclobutyric acid products. Heating at 180°C as a precursor of o-quinone dimethane5awith A solution of N-methylmaleimide in dichlorobenzene provided the Diels-Alder adduct in 60% yield.5aIt can be easily converted to amines of important medicinal value. Benzocyclobutylamide is a patented crop protection bioactive molecule that can be protected by fatty acids4aandRapid synthesis of [2 2] cyclization of N-dihaloarylamides.
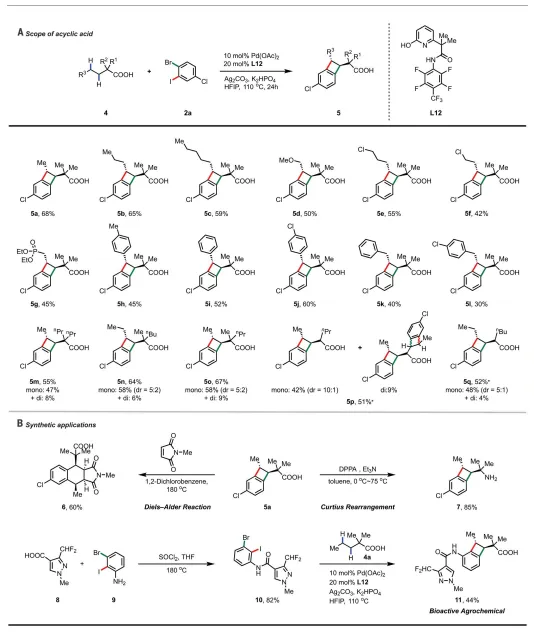
Figure[2 2] Range of acyclic fatty acids for cyclization reactions and product transformations
Dihaloaromatic Substrate Range
The broad range of bromoiodoaromatics was further expandedDiversity of BCBs. Substituents in different positions are compatible with the catalytic system. Halogens such as fluorine, chlorine and bromine are compatible and provide the desired BCB in good yield. Also for the electron-giving (12k-12l) and electron-withdrawing (12m-12t) The aromatic hydrocarbon of the group was tested and provided a good yield for the product. Widely used in C- H activated amides (12s-12t) and carboxylic acids (12u) group as a guiding group is also suitable for this scheme. Aryl triflates are highly reactive sites in Pd cross-coupling chemistry and are also tolerable (12m). In addition, dibromoaromatics and diiodoaromatics are suitable for standard conditions to produce the desired BCB in moderate to good yields. As expected,1bwith 2,3-Dibromotoluene (2cBr) The reaction provides a mixture of two regioisomers, indicating that bromoiodoarenes with two different active sites are essential for controlling regioselectivity.
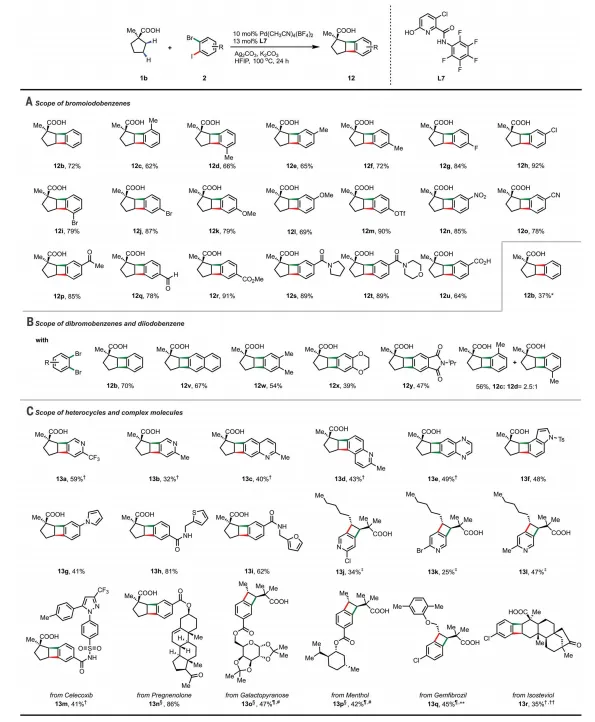
Figure[2 2] Range of Dihaloarenes for Cyclization Reactions
Heteroaromatic hydrocarbons and complex molecules and mechanism studies
The authors found that heterocyclic compounds such as pyrrole, thiophene and furan are well tolerated and can provide the desired product in excellent yield. Dihaloarenes with bioactive structures such as celecoxib, pregnenolone, galactose and menthol are also compatible. This protocol can also be applied to the late modification of bioactive molecules.TwoSequential activation of C- H bonds and two aryl halide bonds with unexpected exclusive regioselectivity.
OutlookAnyway,The authors investigated the palladium-catalyzed double of two adjacent methylene units in carboxylic acidsC- H activation, to achieve a controlled synthesis of BCBs.In addition to achieving two adjacent methyleneIn addition to the regioselective functionalization of C- H bonds, the modular synthesis of multiple BCBs with unique regioselectivity is suitable for pharmaceutical, material science, mechanochemistry, and natural product synthesis. Further studies on the reaction mechanism are underway.
References:JI-MIN YANG, et al. Regio-controllable [2 2] benzannulation with two adjacent C(sp3)–H bonds. Science, 2023, 380(6645): 639-644.DOI: 10.1126/science.adg5282https://www.science.org/doi/10.1126/science.adg5282

Previous article
RELATED INFORMATION

Contact us
Website: www.amoycat.cn
Address: No. 66 Xinyuan South Road, Haicang District, Xiamen City, Fujian Province, China

Official account QR code






