Acid OER, aboard the Science!
2024-08-02
low temperature water electrolysisEnvironmentally sustainable or green hydrogen can be produced quickly

research background
low temperature water electrolysisEnvironmentally sustainable or green hydrogen can be produced quickly, and is a promising way to store energy from renewable but intermittent sources such as wind and solar energy in future clean energy infrastructure. Compared with alkaline electrolyzers, proton exchange membrane electrolyzers(PEMWE) with higher current density, H2The advantages of higher purity, lower resistance loss, and more compact design are the preferred technology for high efficiency and small footprint.
Key issues
however,PEMWE heavy water electrolysis still has the following problems:
1. The activity and stability of the catalyst are the key factors that limit the efficiency of water electrolysis.The high demands on the activity and stability of catalysts operating in acidic and oxidizing environments add to the challenges of water electrolysis. Especially for the oxygen evolution reaction(OER), this is especially the case for the anode catalyst.
2. The high cost of existing catalytic materials limits the wide application of PEMWE.Currently, it is usedThe OER catalysts of PEMWE are mainly limited to platinum group metal (PGM) materials, however, their high cost and limited reserves severely limit the wide application of PEMWE.
3. Low-cost catalytic electrode has limited catalytic performance in acidic electrolyteLow-cost transition metals and their oxides in alkaline electrolytesOER are active, but their performance in acidic electrolytes is very limited.
4, emerging cobalt-based catalysts inApplications in PEMWE still need to be exploredEmerging cobalt-based catalysts have been shown to have high catalytic activity, but the presence of metal precursors often hinders the integration into In PEMWE, and most of the research has been carried out in half-cells or aqueous electrolyzers, the performance in PEMWE needs to be verified in an operating electrolyzer.
new ideas
in view of this,Argonne National LaboratoryBy-Jia Liuet al. reported a nanofibrous cobalt spinel catalyst with lanthanum(La) and manganese (Mn) codoped, the catalyst is prepared from a zeolite imidazole salt framework embedded in electrospun polymer fibers.The catalyst in10 mA/cm2When showing Low overpotential of 353 mVand in an acidic electrolyteThe OER decay rate is low in 360 hours. PEMWEs containing this catalyst at the anode have a current density of 2000 mA/cm at 2.47V(Nafion 115 membrane) or 3.00V(Nafion 212 membrane)2, And the decay rate in the accelerated stress test is low.
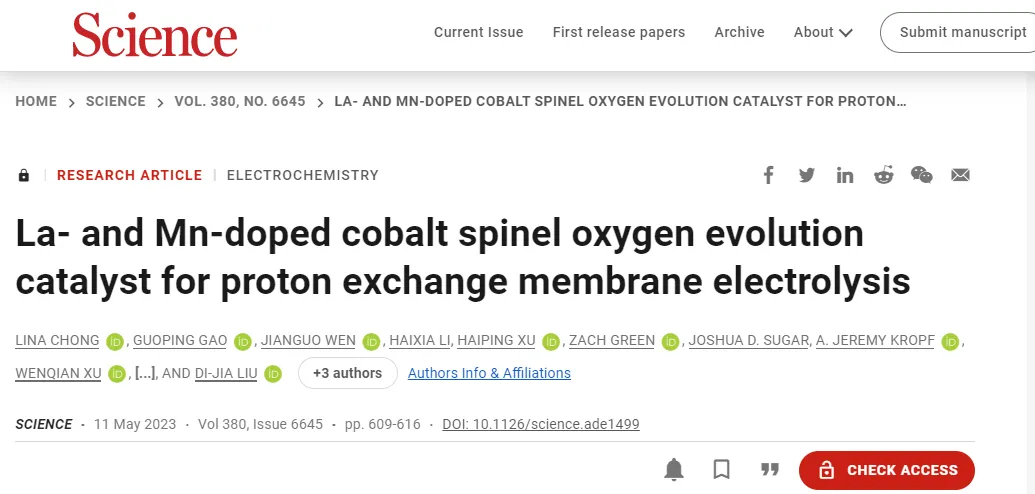
Technical scheme:
1. Design and synthesis of an acid-stabilized cobalt OER catalystThe authors designed a methodThe cobalt spinel-based high-efficiency OER catalyst for PEMWE anode, Co-ZIF was selected as the precursor for catalyst preparation, and La and Mn doped LaMn @ Co-ZIF were prepared by electrospinning.
2. The electronic configuration and coordination structure of the catalyst were studiedAuthors useThe electronic configuration and coordination structure of Co, La and Mn in LMCF were studied by XPS and XAS. confirmed the existence of high V in LMCFoConcentration, distortedCo oxide lattice, and the coordination numbers of Mn with O and Mn with Co were determined.
3, characterized.Electrocatalytic Activity of LMCFThe authors are in aqueous solution andThe OER catalytic activity of LMCF was tested in PEMWE, and the results showed that the OER activity was gradually improved after the addition of Mn and La, and the catalyst had excellent stability.
4. Analysis of the catalytic active siteThe author passes onIn situ XAS tests of LMCF, which analyzed the nature of the active site, showed that Mn and La were not directly involved in electrocatalysis, and their presence changed the structure and activity of the cobalt site.
5. The theoretical calculation once again confirms the experimental results.The author confirmed.The experimentally observed atomic and electronic configurations in LMCF are in good agreement with DFT calculations, analyzing the origin of LMCF activity and conductivity.
Technical advantages:
1. Cobalt spinel catalyst was obtainedThe authors propose a method based on cobalt spinelOER catalyst, which was derived from a zeolite methylimidazolyl ester framework (Co-ZIF) and processed by electrospinning.
2, obtained excellentOER activityThe catalyst prepared by the author has its high specific surface area, porous interconnected nano-network structure and high conductivity, and exhibits excellentOER activity.
Technical Details
an acid-stabilized cobaltDesign of OER CatalystForThe design concept of the high-efficiency OER catalyst based on cobalt spinel for PEMWE anode is based on the following basic principle: selectively introduce an oversized and more stable second element into the cobalt oxide surface to generate strain, oxygen vacancy (Vo) and acid resistance to improvein acidOER activity; A third element with a charge and size similar to cobalt is incorporated into the lattice, through whichd-orbital partial occupancy caused by d-electron delocation to bridge the Fermi band gap to improveConductivity of oxides; The catalyst should have high porosity and easy access to reactantssurface area, the electrode layer should effectively transport H2O and Release O2; metal oxides in The PEMWE environment shall be stable against oxidation and acid corrosion. Based on this, the author chose Co-ZIF as the precursor of catalyst preparation, and prepared La and Mn doped Co-ZIF,LaMn @ Co-ZIF(LMCF) by electrospinning.
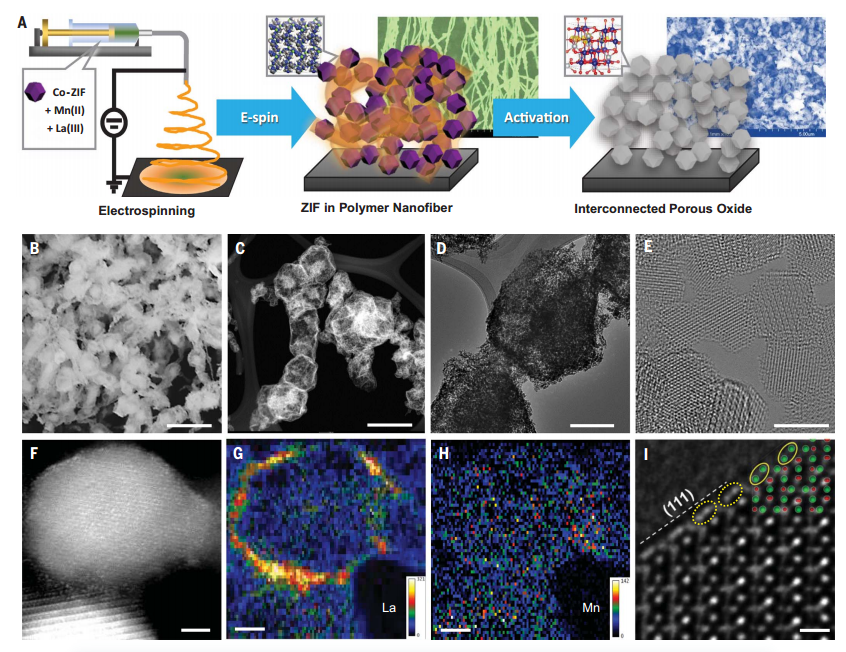
FigureSynthesis, morphology and structure of LMCF
characterization of catalyst microstructureUseThe electronic configuration and coordination structure of Co, La and Mn in LMCF were studied by XPS and XAS. High-resolution XPS spectra confirm the presence of high V in LMCFoconcentration, andCo3O4compared,LMCF中Co2 :Co3 The proportion is higher,The average oxidation state of cobalt in LMCF is low and the O coordination number (CN) is small. Enhanced 1s → 3d transition peak intensities indicate that cobalt in LMCF is more than Co3O4The cobalt in is in a more central symmetric coordination environment, indicating that Vodistorted.Co oxide lattice. The average manganese oxidation state is between 3 and 4. The R space fit determined that the CN of Mn and O and Mn and Co were 5.5±0.4 and 7.2±0.3, respectively.
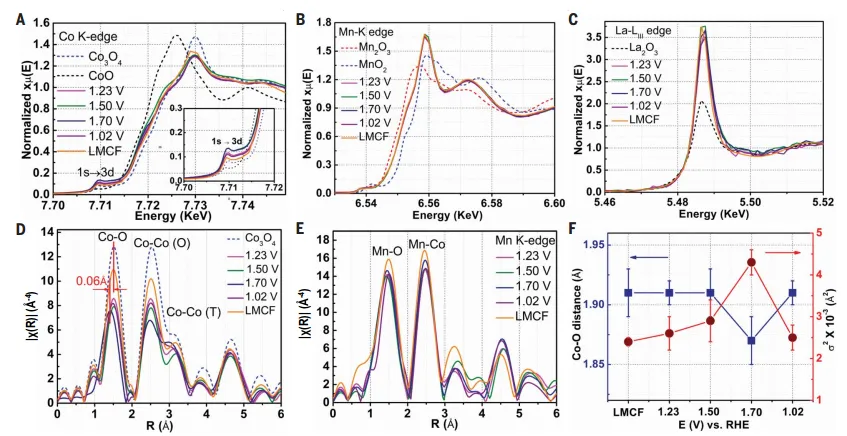
FigureXAS study of LMCF
solution neutralizationElectrocatalytic activity in PEMWEFirst useRDE在 0.1 M HClO4The OER catalytic activity of LMCF was evaluated in the electrolyte. The results showed that by adding Mn and La to CF, the OER activity was gradually improved, and the performance of LMCF also significantly exceeded that of commercial Co3O4and close to Ir, showing high intrinsic catalytic activity. In addition, LMCF also exhibits high FE and Chang cycle life in aqueous solution. Then, the author tested the performance of LMCF in the running PEMWE. The LMCF was assembled into the anode of the PEMWE single cell, and the electrolyzer reached 2000 mA cm at a voltage of 2.47V.-2The current density. The authors also confirmed the excellent catalyst stability of this catalyst.
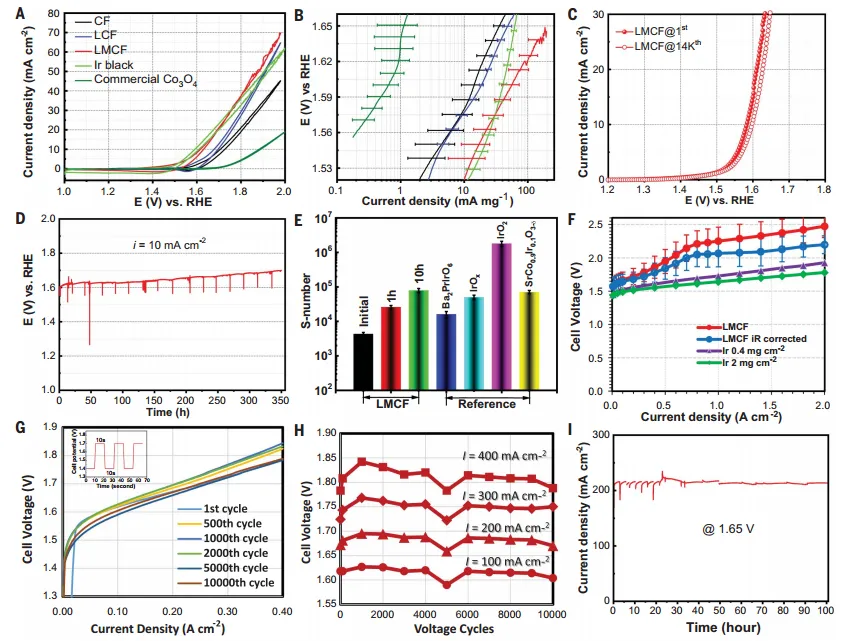
FigureElectrocatalytic Performance of LMCF
active site analysisTo understand the nature of the active site and the effect of second and third metal doping, inO2In the saturated electrolytic cellIn-situ XAS tests of LMCF were carried out for Co, Mn K side and La-LIII side. It is shown that the centrosymmetric coordination (symmetry breaking) and electronic structure of cobalt are altered by oxygen loss and structural changes due to interaction with an acidic electrolyte under an electric field. CN is significantly lower than Co3O4, indicating that tetrahedral coordinationCo2 The proportion is higher, at the same timeVohigher concentration, as a promotionNucleophilic sites for O-O bond formation. With the adsorption of H2O to O2,Co-O vibrations increase, which may involve reconstructed lattice oxygen in the LMCF. The participation of surface and lattice oxygen produces an anisotropic shift in the connection with cobalt, and the recombination of the active site in LMCF is reversible.Mn and La are not directly involved in electrocatalysis, and their presence changes the structure and activity of the cobalt site.
theoretical comparisonThe experimentally observed atomic and electronic configurations in the LMCF are in good agreement with density functional theory (DFT) calculations. The calculations show that, in the ensemble, Mn and La preferentially replace Co in octahedral sites in terms of total energy, in agreement with those observed by XAS described above. Mn is preferentially retained in Co3O4in the main body, andLa is extruded to the surface due to its large size. According to the design concept, the role of dopants in enhancing oxide conductivity and surface defects or oxygen vacancies to achieve better OER activity was confirmed. To better understand the enhanced acid resistance of LMCF under electrolytic conditions, the La-doped Co3O4(111) Surface Pourbaix diagram, the calculated Pourbaix diagram shows that the stability of the LMCF catalyst is determined by the combination of battery potential and pH. LMCF calculations show that with Co3O4uniformly distributed in the latticeMn3 ions instead of low concentrationsCo3 The ions give rise to two partially occupied defect states in the mid-band gap, directly enhancing the bulk-based electronic conductivity.
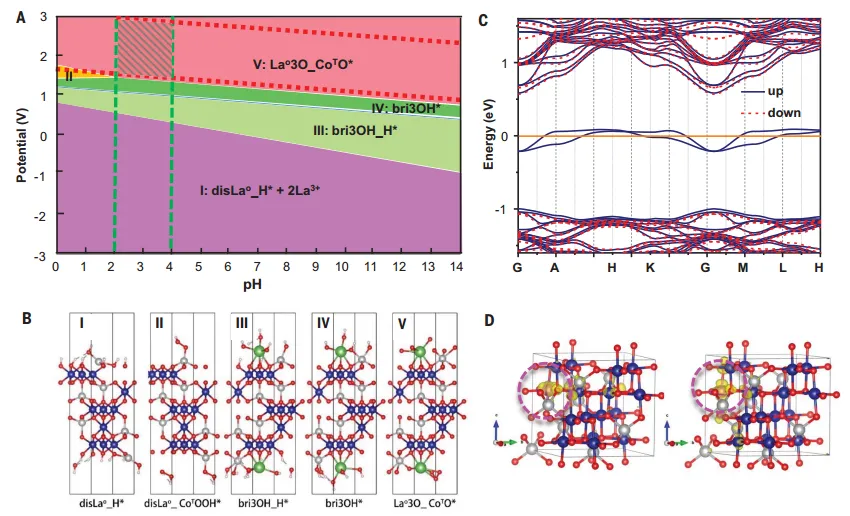
FigureCalculated Pobiplot and Fermi band structure of LMCF
OutlookAnyway,This study is for future usePEMWE Technology Development of PGM-Free OER Catalysts for Hydrogen Production Offers Forward-Looking Direction and Design Insights. For example, increasing the surface functional group density through elemental doping, primary size control, and morphological innovation can further enhance catalytic activity.The durability of PEMWE can be improved by removing the electrochemically unconnected oxide. A basic understanding of the OER mechanism of mononuclear and binuclear reaction intermediates and catalytic pathways will help guide precursor and catalyst design to achieve lower overpotential and better acid resistance. These improvements provide a pathway to the next generation of PGM-free OER catalysts.

In 2018,Argonne National LaboratoryBy-Jia LiuAs the corresponding author, published low-cost fuel cell catalyst research results, the first author is stillLina Chong 。
References:LINA CHONG, et al. La- and Mn-doped cobalt spinel oxygen evolution catalyst for proton exchange membrane electrolysis. Science, 2023,380(6645):609-616.DOI: 10.1126/science.ade1499https://www.science.org/doi/10.1126/science.ade1499

RELATED INFORMATION

Contact us
Website: www.amoycat.cn
Address: No. 66 Xinyuan South Road, Haicang District, Xiamen City, Fujian Province, China

Official account QR code






